19. October 2021
Naviplan FDA 510k Clearance
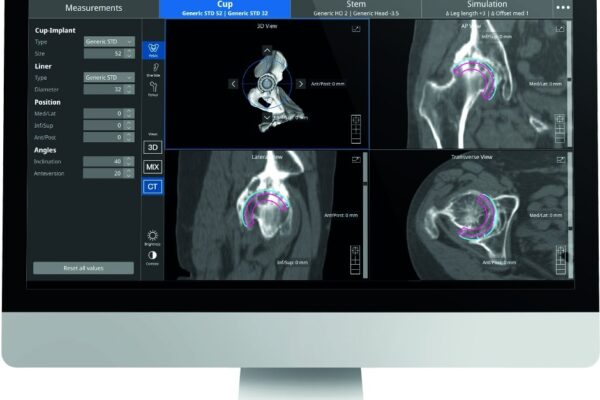
Naviswiss gained 510k clearance from the FDA to market Naviplan in the United States. Naviplan, the digital pre-operative planning solution enables orthopedic surgeons to perform navigated CT-based total hip replacement surgery. It has the potential to improve accuracy and predictability and provides a seamless documentation of the outcome.
As the technology leader in miniaturized surgical navigation solutions, Naviswiss supports orthopedic surgeons in accurately positioning hip replacement implants. The Naviplan hip application is CT-Based and assists the surgeon in the optimal positioning of the joint implants, automatic 3D segmentation and advanced image processing.
CT-based Navigation and Naviplan will be released to orthopedic care centers in the United States over the course of the fourth quarter 2021.